Introduction: SMM is a precursor disorder to multiple myeloma (MM) (Rajkumar SV, et al. Blood. 2015;125[20]:3069-3075). Current guidelines for SMM recommend active monitoring, with treatment initiation only upon progression to MM. However, therapeutic intervention at the SMM stage may help delay the progression to MM (Lonial S, et al. J Clin Oncol. 2020;38[11]:1126-1137; Mateos MV, et al. ASH 2022. Abstract 118). DARA is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. Given the deep and durable responses and favorable safety profile of DARA monotherapy in patients (pts) with relapsed/refractory MM (Usmani SZ, et al. Blood. 2016;128[1]:37-44), we hypothesized that DARA could delay the progression of SMM to MM. In the primary analysis of the phase 2 CENTAURUS study (NCT02316106), after a 15.8-month median follow-up, DARA monotherapy showed activity and was well tolerated in pts with intermediate- or high-risk SMM (Landgren CO, et al. Leukemia. 2020;34[7]:1840-1852). Activity and tolerability of DARA monotherapy were also observed in CENTAURUS after an additional 10 months of median follow-up (Landgren CO, et al. ASH 2018. Abstract 1994). Here, we present the final analysis of the CENTAURUS study, with a median follow-up of 85.2 months (~7 years).
Methods: Eligible pts had a diagnosis of intermediate-risk or high-risk SMM for <5 years. Pts were randomized (1:1:1) to receive 8-week cycles of DARA 16 mg/kg intravenously (IV) on 1 of 3 dosing schedules. In the Intense dosing arm, pts received DARA QW (Cycle 1), Q2W (Cycles 2-3), Q4W (Cycles 4-7), and Q8W (Cycles 8-20). In the Intermediate dosing arm, pts received DARA QW (Cycle 1) and Q8W (Cycles 2-20). In the Short dosing arm, pts received 1 cycle of DARA QW. For pts in the Intense and Intermediate arms, there was an option to extend treatment (Extension phase) with DARA IV or with subcutaneous DARA (DARA SC; DARA 1,800 mg co-formulated with recombinant human hyaluronidase PH20 [2,000 U/mL; Halozyme, Inc.]) Q8W after the end of Cycle 20 per investigator discretion if there was a positive benefit/risk ratio, no grade ≥3 treatment-related toxicity, and at least stable disease had been achieved. Rate of complete response or better (≥CR) was a primary endpoint. Secondary endpoints included overall response rate (ORR) and overall survival (OS).
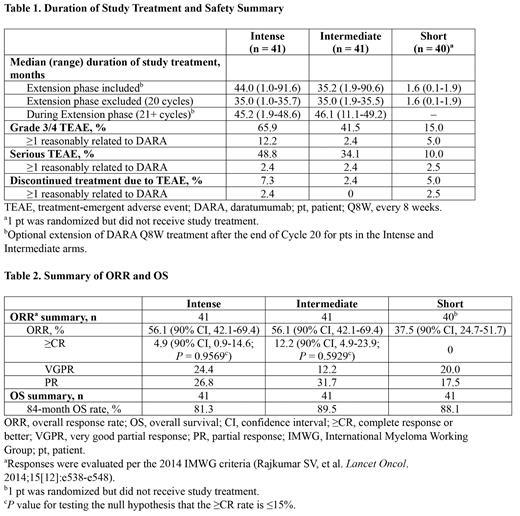
Results: A total of 123 pts (41 pts per arm) were randomized. Median (range) age was 61 (31-81) years. Median duration of study treatment overall during the study was 44.0 months in the Intense arm, 35.2 months in the Intermediate arm, and 1.6 months in the Short arm. A total of 36 pts (21 pts in the Intense arm and 15 pts in the Intermediate arm) continued to receive DARA Q8W in the Extension phase (Cycles 21+). During the Extension phase, median duration of study treatment was 45.2 months in the Intense arm and 46.1 months in the Intermediate arm ( Table 1). 16 pts in the Intense arm and 10 pts in the Intermediate arm switched from DARA IV to DARA SC during the Extension phase.
Efficacy results are summarized in Table 2. At a median (range) follow-up of 85.2 (0-94.3) months, ORR and ≥CR rate were higher in the Intense and Intermediate arms than in the Short arm. In the Intense and Intermediate arms combined, the ≥CR rate was 8.5%. Median OS was not reached in any dosing arm; the 84-month OS rate was 81.3%, 89.5%, and 88.1% in the Intense, Intermediate, and Short arms, respectively.
Safety results are summarized in Table 1. Grade 3/4 treatment-emergent adverse events (TEAEs) occurred in 65.9%, 41.5%, and 15.0% of pts in the Intense, Intermediate, and Short arms, respectively; grade 3/4 TEAEs were reasonably related to DARA in 12.2%, 2.4%, and 5.0% of pts, respectively. TEAEs that led to discontinuation of DARA occurred in 7.3%, 2.4%, and 5.0% of pts in the Intense, Intermediate, and Short arms, respectively.
Conclusions: Findings from this final analysis of CENTAURUS continue to demonstrate the clinical activity of DARA monotherapy in pts with intermediate- or high-risk SMM after a median follow-up of ~7 years. A high proportion (44%) of pts in the Intense and Intermediate arms completed 20 cycles and remained on DARA monotherapy per investigator discretion, with a median duration of additional treatment of 46.0 months (~4 years) in the Extension phase. No new safety concerns were observed with extended follow-up.
OffLabel Disclosure:
Landgren:Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Adaptive: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Theradex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees. Chari:Antengene: Consultancy; Seattle Genetics: Other: Advisory Board, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Secura Bio: Consultancy, Other: Advisory Board; Shattuck Labs: Other: Advisory Board; BMS: Consultancy, Other: Advisory Board, Research Funding; AbbVie: Other: Advisory Board; Janssen: Consultancy, Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board; Glaxo Smith Kline: Other: Advisory Board; Sanofi: Other: Advisory Board; Karyopharm: Other: Advisory Board; Amgen: Consultancy, Other: Advisory Board, Research Funding. Cohen:Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Spencer:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria; Antengene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; IDP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Voorhees:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Nervianos Medical Sciences: Research Funding; Regeneron: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Novartis: Consultancy; GSK: Consultancy, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring. Sandhu:Janssen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Forus: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Jenner:Janssen, BMS, Pfizer, Sanofi: Consultancy, Honoraria. Smith:Janssen, Abbvie, Takeda, BMS, Menarini, Sanofi: Honoraria, Other: Support for conference travel and attendance. The research funding referred to above is agreed inprinciple but has not yet been received and may not be until 2024., Patents & Royalties: Honoraria for speaking, assiting in training events, Research Funding. Cavo:Janssen: Consultancy, Honoraria, Speakers Bureau; GlaxoSmithKline: Honoraria; Adaptive: Honoraria; Celgene/Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Takeda: Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Honoraria; Roche: Honoraria. van de Donk:Abbvie: Other: Advisory Board; Pfizer: Other: Advisory Board; Bayer: Other: Advisory Board; Roche: Other: Advisory Board; Takeda: Other: Advisory Board; BMS: Other: Advisory Board, Research Funding; Cellectis: Research Funding; Novartis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; Amgen: Other: Advisory Board, Research Funding; Janssen Pharmaceuticals: Other: Advisory Board, Research Funding; Servier: Other: Advisory Board; Adaptive: Other: Advisory Board. Beksac:Menarini: Other: Advisory Board; Janssen: Other: Advisory Board, Speakers Bureau; Pfizer: Other: Advisory Board; Amgen: Speakers Bureau; BMS: Speakers Bureau; Takeda: Speakers Bureau. Moreau:janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards; GSK: Honoraria, Other: Advisory Board. Goldschmidt:Morphosys AG: Research Funding; Glycomimetics: Research Funding; GSK: Honoraria, Other: Travel Support, Research Funding; Millenium Pharmaceuticals: Research Funding; KaryoPharm: Research Funding; Array Biopharma: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Heidelberg Pharma: Research Funding; Incyte: Research Funding; Hoffman- La Roche: Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Johns Hopkins University: Research Funding; Pfizer: Honoraria, Patents & Royalties: Travel Support, Research Funding; Takeda: Research Funding; MSD: Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Novartis: Honoraria, Other: Travel Support, Research Funding; Molecular Partners: Research Funding; Mundipharma: Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Chugai: Honoraria, Patents & Royalties, Research Funding; Dietmar-Hopp-Foundation: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding. Sha:Johnson and Johnson: Current Employment. Li:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Rousseau:Janssen: Current Employment. Dennis:Janssen Research & Development: Current Employment. Carson:Janssen Research & Development, LLC: Current Employment. Hofmeister:Sanofi: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pfizer: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees.
Daratumumab monotherapy is currently approved for the treatment of patients with relapsed/refractory multiple myeloma, but it is not yet approved for the treatment of patients with smoldering multiple myeloma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal